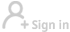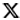Vaccine distribution's success depends on cooperation

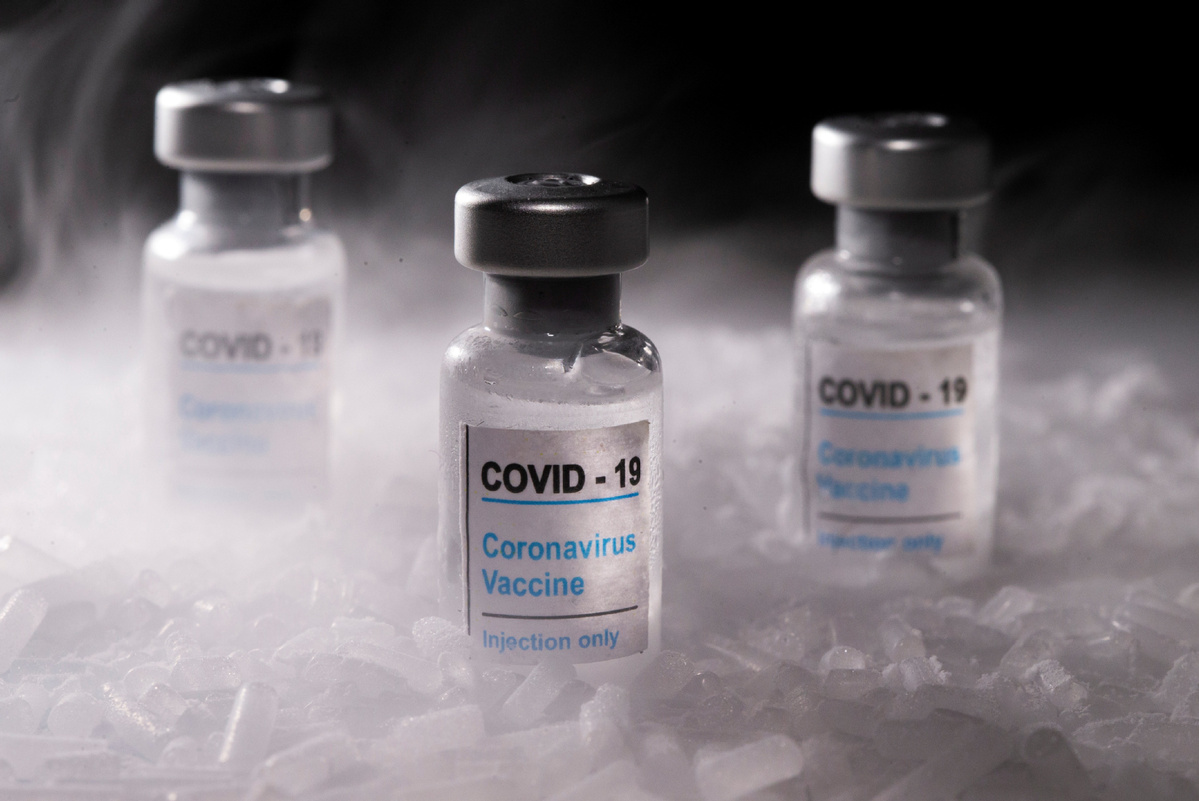
With several COVID-19 vaccines almost ready to go around the world, the focus is shifting to the next giant problem of distribution.
In some ways, developing the vaccine was the easy part-nobody had any doubts that vaccines had to be developed. Getting vaccines to billions of people quickly and efficiently may prove to be a very difficult task altogether. This would be tricky under the best conditions, and the urgency of the current situation gives rise to many problems and multiple difficult decisions.
One immediate and very practical challenge is the sheer size of the logistical operation required to distribute the estimated 12 billion to 15 billion doses of COVID-19 vaccines that will be necessary to return the world to normal. Two-thirds of these doses should be ready through 2021. The World Health Organization estimates that as much as half the vaccine production could be wasted every year due to the challenges of temperature control, logistics and shipment issues.
The closest similar exercise may be the annual distribution of flu vaccines. Every year, about 6.4 billion doses of flu vaccines are manufactured and distributed globally. But these vaccines are easier to store, distribute and administer, and the supply chains are already in place.
Distributing COVID-19 vaccines will be an unprecedented logistical operation. Vaccines will have to be distributed to about 200 countries and to at least 70 percent of the population to achieve something like global herd immunity. Basically, some 5 billion people will have to be vaccinated as quickly as possible.
According to international vaccine alliance Gavi, the key obstacle to distributing a vaccine will be supply constraints.
The cost alone will be staggering. A recent delivery of vaccines from China to Brazil cost more than $1 million.
A key consideration is that a few of the vaccines that are almost at the approval stage in the United States and Europe require extremely cold temperatures to remain stable. The vaccine developed by Pfizer and BioNTech that the United Kingdom approved for emergency use on Dec 2 and the US could approve as early as Thursday has to be kept and distributed at minus 70 C and requires two shots administered three weeks apart. Moderna's vaccine, which could get emergency approval at the same time, also requires two shots and has to be kept at minus 20 C.
As a side note, China's Fosun International bought $50 million in BioNTech stock and provided $85 million toward developing the company's vaccine. Shanghai Fosun Pharmaceutical and Sinopharm Group are working together to create a cold chain to get the vaccine across the Chinese mainland and the Hong Kong and Macao special administrative regions.
Countries with less developed or weaker infrastructure will simply not be able to use these vaccines.
A vaccine under development by AstraZeneca and Oxford University can be stored at room temperature, but there is only so much of that vaccine that can be produced over the next few months.
Some of China's vaccines that should be approved over the next month or two should also be easier to distribute. Sinovac said its CoronaVac may remain stable for up to three years in storage. In early trials, the vaccine remained active for 42 days at 25 C or five months at 2 C to 8 C, the company said. The vaccine being developed by CanSino is also more stable.
Logistics companies from around the world, not least from China, are working on the distribution challenges.
Chinese logistics companies are already laying the groundwork to distribute vaccines at home and around the world. Given China's manufacturing and logistics capabilities, the country is likely to play a key role in global distribution, particularly among allies in Asia. Vaccine makers with products close to market-Sinopharm, CanSino and Sinovac-h(huán)ave begun working on distribution plans.
CanSino is sending the raw material of its vaccine in cans as opposed to individual shots, according to reports. All the vaccine makers are seeking export licenses, even before the products are approved. Shipments could start as early as mid-December.
Companies in the logistics business are stepping up to help resolve the challenges.
China's SF Express is distributing equipment to ensure the integrity of cold chains wherever it distributes.
China International Marine Containers has developed a vaccine shelter with cold storage, positive pressure and inactivation systems to transport vaccines across long distances and avoid such challenges as power outages.
Qin Yuming, the secretary-general of the Cold Chain Logistics Committee of the China Federation of Logistics and Purchasing, told the media that China has taken a global lead in cold-chain transportation capabilities over the past five years, thanks to new standards and regulations.
As with the development of a vaccine, there are solutions for this huge distribution problem. The solutions will require ingenuity and international cooperation.
The author is managing director of Bahati Ltd, a Hong Kong-based editorial services consultancy.