Valneva COVID vaccine approved for use in Britain

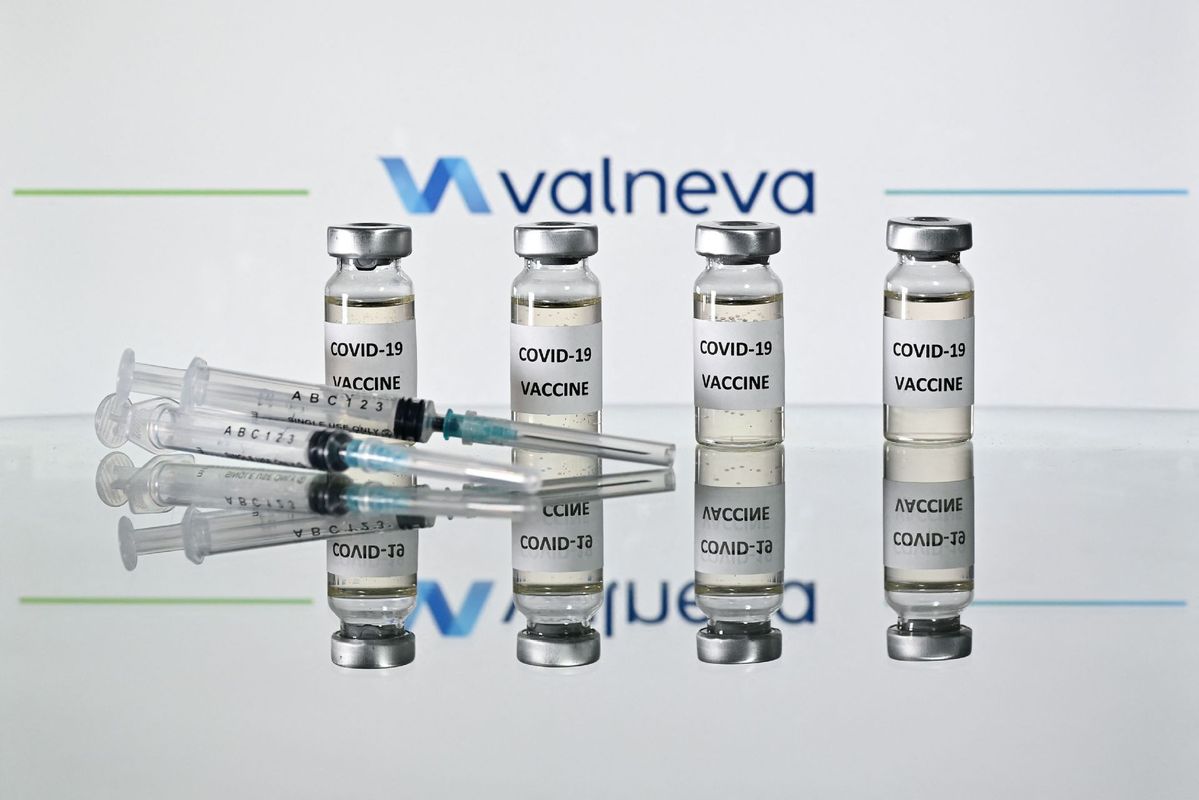
LONDON - A COVID-19 vaccine developed by the French firm Valneva was granted regulatory approval on Thursday by the British Medicines and Healthcare products Regulatory Agency (MHRA).
The Valneva jab is the first whole-virus inactivated COVID-19 vaccine to gain regulatory approval in Britain.
The vaccine is approved for use in people aged 18 to 50 years, with the first and second doses to be taken at least 28 days apart.
The British independent medicines regulator is the first in the world to approve the Valneva product, MHRA said in a statement. It is also the sixth coronavirus vaccine to be granted an MHRA authorization.
Britain reported 238,938 COVID-19 cases and 1,984 deaths in the past seven days. The caseload in Britain has reached 21,747,638, with total deaths of 171,396, official figures on Thursday showed.
More than 92 percent of people aged 12 and above in Britain have had one shot of COVID-19 vaccine, more than 86 percent have received both shots and more than 67 percent have received a booster jab.